Summary
In this laboratory, an unknown ore will be dissolved in HCl, complexed with 1,10 phenanthroline, and analyzed by UV-vis spectroscopy.
Introduction
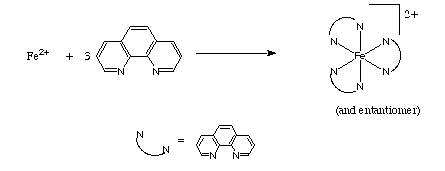
Iron(II) solutions react with 1,10 phenanthroline (phen) to form [Fe(phen)3]2+ salts.

Solutions of these iron salts have intense absorbtions in the visible region that allows the determination of the concentration from Beer's Law.
Part 1. Constructing a Calibration Curve
Things that need to be done:
(1) Dissolve 100 mg of 1,10 phenanthroline monohydrate in 100 mL of water. Store in a plastic bottle.
(2) Dissolve 10 g of hydroxylammonium chloride in 100 mL of water
(3) Dissolve 10 g of sodium acetate in 100 mL of water
(4) Add about 70 mg of Fe(NH4)2(SO4)2 • 6H2O and 2.5 mL of concentrated sulfuric acid to a 1000 mL volumetric flask. Dilute this to the mark with DI water. Calculate the concentration of this standard in ppm.
Place 1, 2, 5, 10, and 25 mL (measured by volumetric pipettes) of the standard iron solution (prepared in 4 above) into a series of 100 mL volumetric flasks. Add 1.0 mL of the hydroxylammonium chloride, 5.0 mL of the phenanthroline solution, and 8.0 mL of the sodium acetate solution to each of the flasks and dilute to the mark with water. Mix the solutions and allow to stand for 20 minutes. Calculate the concentration in ppm for each of the dilutions.
Take the UV-visible spectrum for each of the standards above. Find the maximum absorbance in the visible region and plot the absorbance vs. concentration.
Part 2. Preparing the Unknown
Solutions to Prepare:
(1) Dissolve 0.6 g of SnCl2•2H2O in 1-2 mL of concentrated HCl (warming may be necessary) and dilute to 10 mL with DI water. Add a few pieces of Sn to help preserve the solution and cap tightly.
(2) Dry the ore for 3 h at 120 degrees C (do the day or week before lab). Cool the ore in a dessicator. Accurately weigh out about 20 mg of the ore into a 100 mL round-bottomed flask. Add 5 mL of concentrated HCl and 2 mL of the SnCl2 solution. Cover the opening of the flask with a watch glass and heat nearly to boiling. Continue to heat until the ore is dissolved (any solid should be white). Dilute the solution to 50 mL in a volumetric flask.
Take the unknown iron solution prepared above and add hydroxylammonium chloride, phenanthroline, and sodium acetate to prepare the iron phenantroline solution. Take the UV-visible spectrum and determine the iron content of the unknown. You may need to dilute the solution prepared in 2 (above) before making the phenanthroline compound.
This experiment was developed by Michael R. Jordan at Oklahoma Baptist University. Reproduction by printing and photocopying for instructional use by educational institutions is permitted.