![]()
(1) All compounds have magnetic properties.
(2) Type of magnetism exhibited depends on the number and arrangement of unpaired electron spins in the molecule or material.
There are different magnetic properties for molecules and for materials.
(3) Molecular Magnetic Properties
-Diamagnetism: Most molecules have all electrons spins paired with opposite electron spins. This is referred to as diamagnetism. Diamagnetic molecules and materials have no permanent magnetic moment. You might expect them to be unaffected by a magnetic field, but they are actually slightly repelled by a magnetic field. This is something to think about with respect to the superconductor lab.
-Paramagnetism: If there are unpaired electron spins in a molecule, it is said to be paramagnetic and has a permanent magnetic moment. This magnetic moment is attracted to a magnetic field.
(4) Magnetic properties of a Material: Cooperative Magnetism
A material made up of paramagnetic molecules or domains containing unpaired electrons can exhibit cooperative magnetic properties. These come in three types; ferromagnetism, antiferromagetism, and ferrimagnetism.
![]()
-Ferromagnetism: If the unpaired spins in the paramagnetic molecules or domains couple so that all of the spins are parallel, the interaction is referred to as ferromagnetic. Ferromagnetic interactions can result in very strong interactions to a magnetic field.
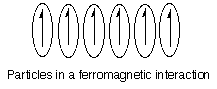
-Antiferromagnetism: This is similar to ferromagnetism except instead of pairing in a parallel fashion, the unpaired electron spins of the particles pair antiparallel (opposite of each other), cancelling the net magnetic moment. This results in weak attraction to a magnetic field even though the individual particles are still paramagnetic.
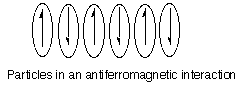
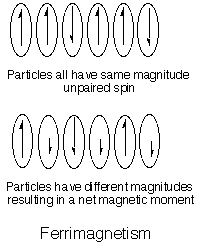
-Ferrimagnetism: In a ferrimagnetic material, the spins of some of the paramagnetic particles are coupled parallel and a few are coupled parallel. Another way to get a ferrimagnetic material is to have two different particles with different spins, coupled in and antiparallel fashion. Examples are shown below.
Return to the main tutorials page