
Magnetic Susceptibility
Calculating Susceptibilities from Balance Readings
Detemining the Number of Unpaired Electrons
The basis behind the magnetic susceptibility balance is that a paramagnetic sample will be attracted to a magnet and a diamagnetic sample will be slightly repelled. In the balance used, the sample is fixed in position and the force of the sample on a pair of permanent magnet is measured yielding the measured magnetic susceptibility.
The magnetic susceptibility initially measured will be Xg, the mass susceptibility. The mass susceptiblity is then converted (usually) to the molar susceptibility by the relation below.
Xm = the molar susceptibility = Xg x MW
In contrast to paramagnetic samples, diamagnetic samples are repelled by the magnetic field. This opposing force is caused by the circulation of paired electrons (caused by the applied magnetic field). Because of this, all atoms with paired electrons have a diamagnetic susceptibility (even if they also contain unpaired electrons). The diamagnetic suseptibility is fairly constant for each atom or functional group and is additive. This means that the diamagnetic susceptibility of a molecule or ion can be approximated by summing the diamagnetic susceptibilities of the atoms or functional groups in the species (Table 1). The diamagnetic susceptibility of the bonds need to be added as well for nonsingle bonds unless the system is aromatic and the aromatic values of the atoms are used.
Example
Calculating the Xdia for NaCH3CO2:
Add up the contributions for the atoms, ions, bonds
C = -6.0
3 x H = 3(-2.93) = -8.79
(O2)- = -6.0
Na+ = -7.0
Correction for the C=O bond= +6.3
Total Xdia = -21.5
These fairly predictable values of the diamagnetic susceptibility are useful because what is measured by the balance is the sum of the paramagnetic susceptibility of the sample (Xpara) and the diamagnetic susceptibility (Xdia). Xpara is usually the value of interest, and can be calculated by subtracting the calculated Xdia from the measured susceptibility (Xmeas).
Xpara = Xmeas - Xdia
Magnetic susceptibilities are temperature dependent. The Curie-Weiss law (below) describes the temperature dependence of many paramagnetic substances.
X = C/(T-A)
X = suseptibility
C= experimental constant
A = intercept (experimental)
Because of this temperature depencence, it is useful to have a measurement of the suseptibility that is temperature independent. This value is called the mu effective and is described by the relation below

The units of mu effective are given in Bohr magnetons (BM). The value of mu effective also allows the calculation of the number of unpaired electons in the compound from the following two relationships for mu effective.
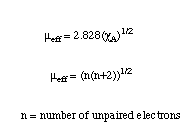
The first of these relations is the same as the equation above it, and the second relates the mu effective to the number of unpaired electrons in the compound. The second equation assumes that there is no spin-orbit coupling in the molecule. This is a fairly good approximation for first row transition metals and lighter atoms, but not a very good one for heavier atoms.

(1) Turn on the machine by turning the RANGE knob from OFF to X1 (measurement times one). The machine needs to warm up for at least 10 minutes.
(2) Your sample should be in the form of a powder. If it is a crystalline sample, crush the crystals into a powder.
(3) Measure the mass of the sample tube (empty) on a mass balance.
(4) Adjust the ZERO knob of the balance until the reading is 000.
(5) Place the empty sample tube carefully into the balance and record the reading. This is R0.
(6) Add a small amount of solid to the tube and gently tap the tube on a solid surface to pack the sample. Continue to add and pack the sample until the length of the sample is between 2 and 3 cm. Record the height of the sample. This height is the measurement, l.

(7) Weigh the packed sample tube on a mass balance. Subtract the reading from the weight of the empty tube to find the mass of the sample.
(8) Place the packed tube into the balance and record the reading, R.
(9) If the measurement goes off scale, remove the sample tube turn the RANGE knob to X10 (measurement divided by 10). Rezero the instrument. Reinsert the sample tube and record the reading. Multiply the reading by 10.
(10) Record the temperature in the room.
(11) It is best to take two measurements of the sample. This should be done by unpacking the tube (or using a second tube), repacking the tube, and repeating the measurements. If the two measurements result is very different measurements of Xg, it may indicate that one of the samples was not packed well.
Calculating Susceptibilities from the Balance Readings
Xg = [CBAL*l*(R-R0)]/(109*m) in c.g.s units where
l = the sample length in cm
m = the mass of the sample in g
R = the reading for the tube plus sample
R0 = the reading for the empty tube
CBAL = the balance calibration constant (written near the balance)
Xm = the molar susceptibility = Xg x MW
Detemining the Number of Unpaired Electrons
Note: This applies only to paramagnetic molecules. If there is cooperative magnetism, this section does not apply.
The measurement taken and the Xm determined is the total susceptibility of the substance. All substances contain a diamagnetic contribution from all of the paired electrons. The paramagnetic contribution comes only from the unpaired electrons. To determine the number of unpaired electrons in the molecue or unit, the diamagnetic contribution must be eliminated. This is fairly simple because the diamagnetic susceptibilities are fairly additive. In table 1, you will find the diamagnetic susceptiblilites for a variety of functional groups, ions, and atoms. Adding all of the diamagnetic susceptibilities for all of the parts of the substance will give you a good approximation for the diamagnetic susceptibility of the substance.
Subtracting the diamagnetic susceptibility from the total magnetic susceptibility, Xm, gives the susceptiblility due to the paramagnetic part of the molecule, XA. The traditional method of listing magnetic susceptibilities is to report the mu effective (below).
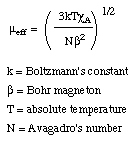
The value of mu effective is listed in Bohr Magnetons (BM). Two other relations for mu effective are as follows.
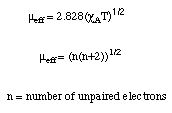
These last two relations allow the number of unpaired electrons to be calculated from the measured paramagnetic susceptibility.
Cooperative Magnetism
If there is cooperative magnetism, a very large magnetic susceptibility can result. Paramagnetism is a property of a molecule. Cooperative magnetism (antiferromagnetism, ferrimagnetism, and ferromagnetism) are properties of bulk materials and there are some differences between these two general types of magnetic behavior. Diamagnetism and paramagnetism do not depend on the strength of the magnetic field, whereas antiferromagnetism, ferrimagnetism, and ferromagnetism do depend on the strength of the magnetic field. Also, ferro- and ferimagnetic materials can have Molar susceptibilities much larger (10 -4 to 10 -2 cgs units) than those of typical paramagnetic species (0 to 10 -4 cgs units).
References:
Drago, R. S. Physical Methods for Chemists, Saunders College Publishing, Orlando, FL, 1992
Table 1. Diamagnetic Susceptibilities
| Atom | Xm (x10-6) | Cations | Xm (x10-6) | Anions | Xm (x10-6) | Bonds and Molecules | Xm (x10-6) |
| H | -2.93 | Mg2+ | -5 | acetate | -29 | C=C | 5.5 |
| C | -6.00 | Fe2+ | -13 | benzoate | -71 | CC triple bond | 0.8 |
| C (aromatic) | -6.24 | Co2+ | -13 | ClO4- | -32 | C=O | 6.3 |
| N | -5.57 | Cu2+ | -13 | CN- | -13 | N=N | 1.8 |
| N (aromatic) | -4.61 | Ni2+ | -13 | CNO- | -23 | C=N | 8.2 |
| N (monamide) | -1.54 | Zn2+ | -15 | CNS- | -34 | CN triple bond | 0.8 |
| N (diamide, imide) | -2.11 | Sr2+ | -16 | (CO3)2- | -28 | N=O | 1.7 |
| O | -4.61 | Ba2+ | -26 | formate | -17 | H2O | -13 |
| O2 (in RCO2R groups) | -7.95 | Cd2+ | -20 | (NO3)- | -19 | NH3 | -16 |
| S | -15.0 | Hg2+ | -36 | (O)2- | -6 | ethylene diamine | -47 |
| P | -26.3 | Pb2+ | -32 | OH- | -11 | pyridine | -49 |
| F | -63 | Li+ | -1 | (S)2- | -28 | triphenyl phosphine | -167 |
| Cl | -20 | Na+ | -7 | (SO4)2- | -38 | ||
| Br | -31 | K+ | -15 | (S2O3)2- | -46 | ||
| I | -44 | Rb+ | -22 | acetoacetonate | -55 | ||
| Cs+ | -33 | ||||||
| NH4+ | -13 | ||||||
| Cu+ | -15 | ||||||
| Ag+ | -27 | ||||||
| Tl+ | -36 |
This page written and maintained by Michael Jordan and Karen J. Brewer